-

ARPANSA releases new regulatory guide
Part of ARPANSA’s role is the regulation of all Commonwealth entities that use radiation. Regulation ensures compliance with the Australian Radiation Protection and Nuclear Safety Act 1998 and associated Regulations (2018) and is supported by a series of regulatory guides. -

No link between mobile phones and brain cancer across all age groups
In December 2018, the British Medical Journal Open published a scientific study led by ARPANSA investigating the relationship between brain cancer and mobile phone use. Since the publication of the original study, the authors have conducted further analysis of additional age groups. -

New ultraviolet radiation sensor now online
We’re pleased to announce that the newest location in our ultraviolet (UV) radiation monitoring network is now online. Emerald is the fourth location in Queensland to join our network of real-time UV data. -
Radiation safety incidents in Australia
ARPANSA collates and publishes an annual report on radiation incidents in Australia. This report is produced with input from various radiation regulators around the country, based on incidents submitted to them and any subsequent investigations they carry out. -

New resource to protect outdoor workers
In Australia, skin cancer accounts for around 80% of all newly diagnosed cancers each year and UV radiation from the sun is one of the leading causes of skin cancer. With high UV radiation year-round in many parts of Australia, people who regularly work outside face a higher risk of developing cancer from sun exposure. -
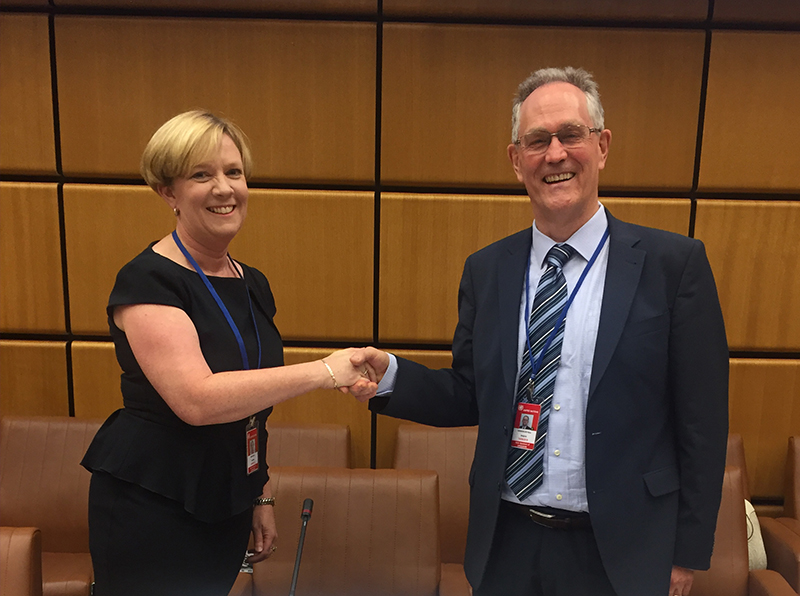
Dr Gillian Hirth elected Chair of United Nations Scientific Committee on the Effects of Atomic Radiation
Dr Gillian Hirth, deputy CEO and Head of ARPANSA's Radiation Health Services Branch, has been elected as Chair of the United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) for the 66th and 67th sessions -

CEO of ARPANSA restricts production in the ANSTO Nuclear Medicine facility after accident
Production of nuclear medicine at ANSTO’s Nuclear Medicine facility was halted on 21 June following an accident in which the hands of three workers were exposed to radiation. -

Contamination event at ANSTO Nuclear Medicine facility
On Friday 21 June 2019, ARPANSA was notified of a radiation contamination event at the Australian Nuclear Science and Technology Organisation (ANSTO) Nuclear Medicine (ANM) production facility in Lucas Heights, New South Wales. -

Misinformation about Australia’s 5G network
ARPANSA is aware that there is a lot of concerning misinformation circulating throughout the community about the possible impacts of Australia’s planned roll-out of the 5G mobile network. -

New emergency exposure guide
We have published the Guide for Radiation Protection in Emergency Exposure Situations. -

ARPANSA authorises ANSTO to commence routine production of molybdenum-99 in the ANM facility
ARPANSA’s CEO, Dr Carl-Magnus Larsson has amended a licence issued to the Australian Nuclear Science and Technology Organisation (ANSTO) to operate the ANSTO Nuclear Medicine (ANM) facility. -

Australian Pilots not at higher risk of melanoma than other Australians
Recently, ARPANSA co-authored a new study which found that modern airline pilots registered in Australia, appear not to be at higher risk of developing invasive melanoma than the rest of the population. -

ARPANSA expands ultraviolet radiation monitoring
We’re pleased to be partnering with the Central Highlands Regional Council to establish Queensland’s fourth location for collection of real time ultraviolet (UV) data. -

ARPANSA authorises limited production of molybdenum-99 in the ANM facility
ARPANSA has granted the Australian Nuclear Science and Technology Organisation (ANSTO) an amended license to allow for the limited production of molybdenum-99 in order to sustain supply. -

5G: the new generation of the mobile phone network and health
At ARPANSA, we set the safety standard sets limits for exposure to radio frequency (RF) electromagnetic energy (EME) that is emitted by mobile phones and other wireless technologies. These limits are set well below levels at which harm to people may occur. The operating frequencies of the 5G network are included within the limits set by the ARPANSA safety standard. -
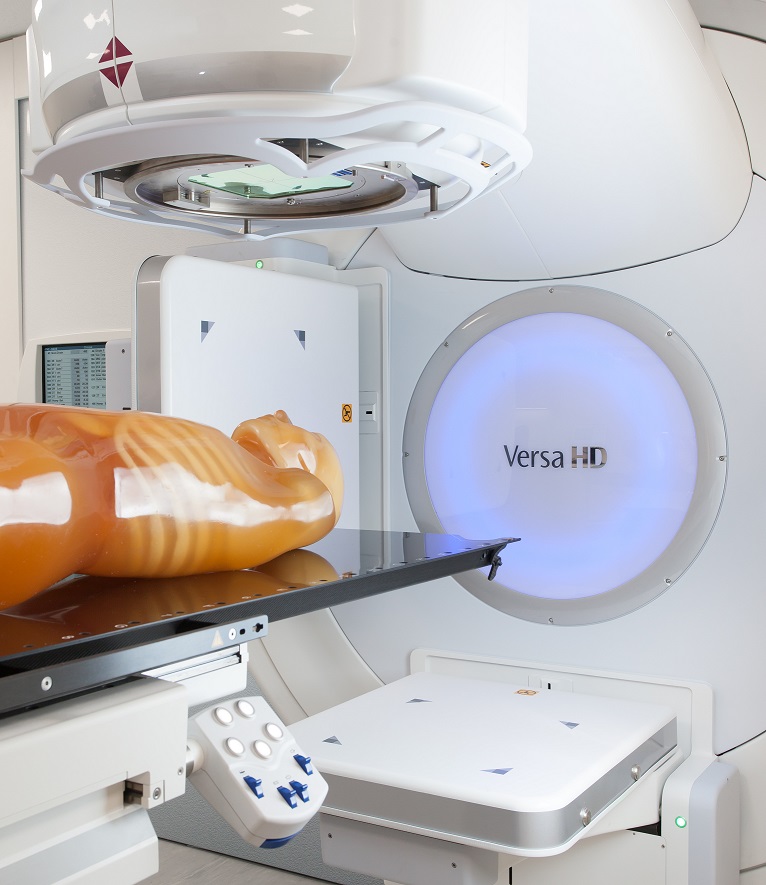
We have installed a new linear accelerator at ARPANSA
We’re excited to announce that we have installed a new linear accelerator (linac) at ARPANSA, to continue ensuring any Australians undergoing medical procedures receive the correct amount of radiation. -

Dr Carl-Magnus Larsson reappointed as CEO for a third term
The Governor-General has reappointed Dr Carl-Magnus Larsson as the CEO of ARPANSA for a term of three years. -

Do you work with or use cosmetic treatments using laser, IPLs or LEDs?
We’ve released national advice for light-based cosmetic treatments in Australia for both patients and practitioners.


